FIRES. As a rare and severe epilepsy syndrome, febrile-infection related epilepsy syndrome (FIRES) is characterized by refractory status epilepticus (RSE) preceded by a febrile illness and often leads to prolonged hospitalizations, cognitive impairment, and intractable epilepsy. There are currently no clear causative etiologies identified in FIRES, and the underlying genetic architecture remains elusive. Here is a brief summary of our recent manuscript on the genetics of FIRES and refractory status epilepticus. This is what we learned about one of the most enigmatic conditions in child neurology.
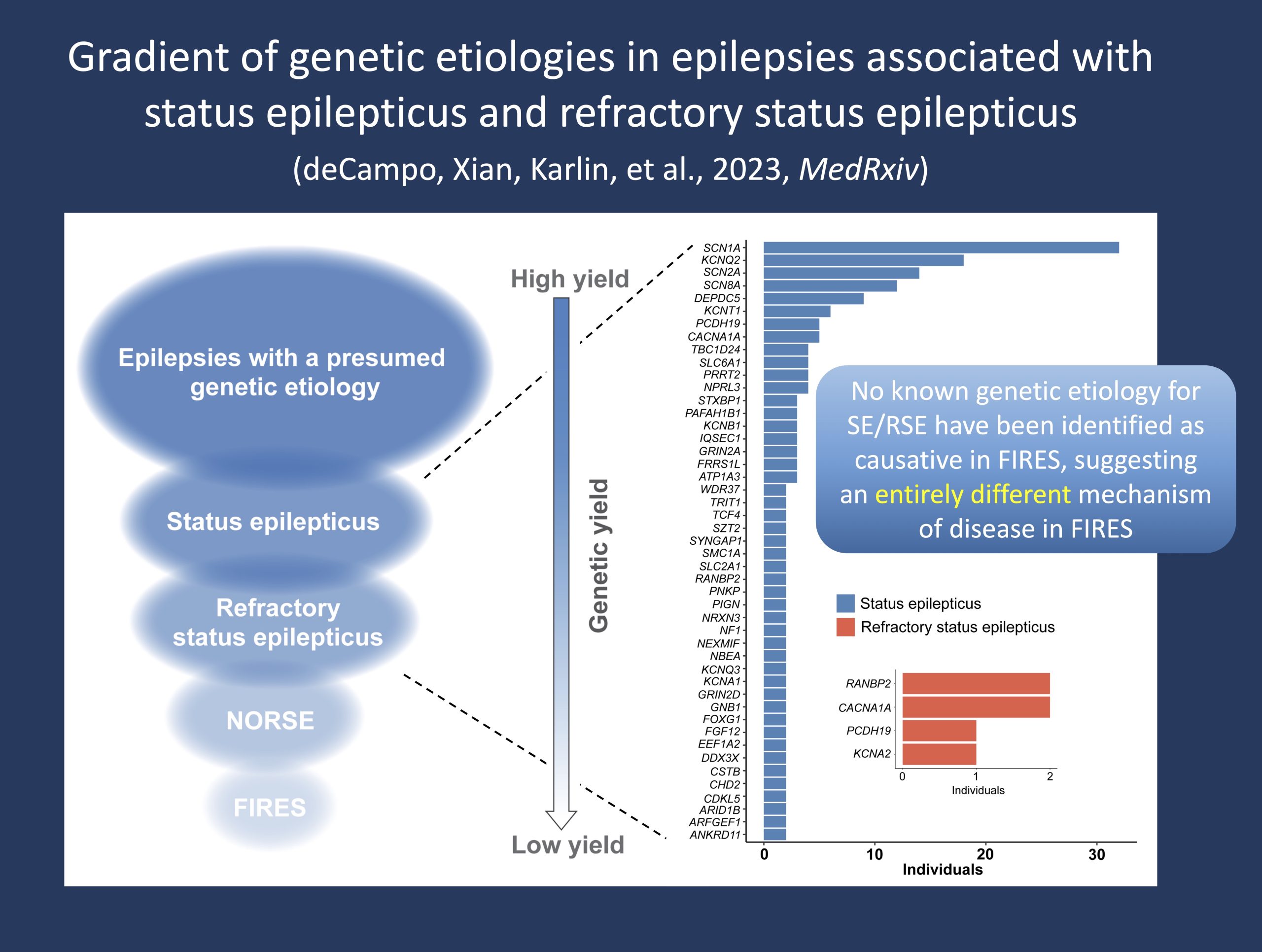
Figure 1. The spectrum of genetic yield in epilepsies with a presumed genetic cause, showing the distribution of identified etiologies in status epilepticus and refractory status epilepticus out of a broader cohort of >2,000 individuals with genetic or presumed genetic epilepsies. We found that the frequency of genetic diagnoses occurs on a gradient, with fewer etiologies associated with more severe presentations including new-onset refractory status epilepticus (NORSE) and a genetic yield of zero in FIRES.
EMR Genomics. In our recent manuscript by deCampo, Xian, Karlin et al, we aimed to understand the genetics of FIRES, new-onset refractory status epilepticus (NORSE), and status epilepticus more broadly using a data mining approach. Given the low population prevalence of FIRES, the first hurdle our team had to overcome was to identify all children with FIRES who had been treated at our center. As a proof of concept, we demonstrate the utility of an EMR Genomics approach by applying natural language processing across millions of free-text patient notes from the last decade within our healthcare network. With subsequent curation, we narrowed our final FIRES cohort to include 25 individuals with a confirmed diagnosis based on formal consensus criteria. With clinical data on all children with FIRES captured across the dynamic landscape of a large pediatric healthcare system and with the earliest diagnosis in 2010, our approach points to how data points in the medical records can be thought of as documents of medical histories. In one of our prior blog posts, we used “phenotypic atoms” to refer to this concept.
Explanatoriness. As of 2023, there remains a genetic yield of 0% in FIRES – not a yield of “almost zero” but a yield of exactly zero with no single individual identified with an explanatory genetic cause. Despite increased comprehensive diagnostic workup, including clinical genetic testing, and despite focused research efforts on surveying the genetic architecture of FIRES, no genetic factors have been identified as causative etiologies. Genetic testing in FIRES has been heterogeneous over the years and even with increased exome sequencing performed as part of routine clinical care, all findings have been non-explanatory. While there have been variants reported in established genes including SCN1A, PCDH19, POLG, DNM1, KCNT1, and SCN2A, none of these variants are considered as an explanation for FIRES. There is a lack of evidence for the explanatoriness of these variants. And yes, the concept “explanatoriness” that we outlined recently made it into the discussion of our paper that is now posted as a preprint.
SE and RSE. To elucidate how FIRES fits within our broader understanding of status epilepticus and RSE, we expanded the scope of our study to assess the genetics of epilepsies associated with SE and RSE (Figure 1). In contrast to FIRES, we identified more than 30 genetic etiologies and a genetic yield greater than 30% in individuals with SE – a yield representative of the broader cohort of individuals with genetic epilepsies. However, while some etiologies such as KCNT1, DEPDC5, and PCDH19 are characterized by status epilepticus in >80% of individuals, the frequency significantly drops when assessing refractory status epilepticus (RSE) as opposed to status epilepticus more broadly. This demonstrates a gradient of etiologies that occurs along a spectrum of epilepsies associated with SE. So, what are the genetic etiologies associated with clinical presentations that most closely resemble FIRES or NORSE?
“FIRES-like” genes. We then aimed to identify etiologies associated with a NORSE or NORSE-like presentation – individuals where refractory status epilepticus occurred without any prior history of seizures. When narrowing our focus to individuals who presented with RSE as the first seizure presentation, only a few genes emerged: CACNA1A, KCNA2, PCDH19, and RANBP2. Of note, two of these individuals met clinical diagnostic criteria for NORSE and had a diagnosis of PCDH19 – what we consider as the most “FIRES-like” gene. Yet, while these findings point to emerging genetic evidence for NORSE, there remains a critical gap in our understanding of FIRES. However, this is not to say that there are no genes underlying the architecture of FIRES – in fact, we have previously shown that there are a range of candidate genes for FIRES. The idea is that gene-disease validity needs to be established before we can consider whether variants are explanatory in a clinical context. This should not mean that genetic testing is not important in FIRES. Quite the opposite, as genetic testing should be an essential part of the work-up given that the full clinical picture is often not clear at onset and identification of genes causative for NORSE, or a FIRES-like presentation, has important treatment implications.
Real-world medical care. In addition to clarifying the role of genetic factors, there is critical value in understanding the clinical care of individuals with FIRES. Thus, an additional dimension of our paper aimed to characterize how real-world medical practice in FIRES has changed in the last decade, starting with the care of a patient with FIRES diagnosed in 2010 to the most recent diagnosis in 2021. Analyzing real-world data (RWD) from our EMR, we found frequent use of steroids and intravenous immunoglobulin (IVIG) and an increased use of immunosuppressants and plasma exchange (PLEX) after 2014. Genetic testing was heterogeneous with regards to testing modality and timing of testing sent relative to disease onset. Therefore, we point to integrating an early and comprehensive genetic work-up following hospital admission as standard of care. Accordingly, we present a picture of past and current clinical decision-making and management practices in FIRES, providing a framework to facilitate improvement of current standards and guide future treatment practices.
What you need to know. In our recent paper, we show how the absence of genetic etiologies in FIRES is in stark contrast to the broader genetic landscape of epilepsies associated with status epilepticus and refractory status epilepticus. This paradigm suggests distinct underlying etiologies and characterizes FIRES as a clinical entity separate from both genetic and other forms of idiopathic SE and RSE. While genetic investigation remains ongoing, it is possible that the underlying etiology is multifactorial and requires collaborative efforts to establish the groundwork for novel diagnostic and future treatment strategies. Nevertheless, alongside continued investigation into the etiology of FIRES, understanding the current treatment landscape and delineating clinical outcomes of individuals with FIRES will remain critical.


