AHC. Amongst the various episodic neurological disorders of childhood, alternating hemiplegia of childhood (AHC) is one of the most mysterious conditions. AHC is characterized by transient hemiplegic attacks and a wide range of other neurological features including dystonic attacks, seizures, neurodevelopmental features, and autonomic symptoms. Recurrent de novo variants in ATP1A3 represent the most common cause of AHC, even though a small subset of individuals have disease-causing variants in other genes. In a recent paper, de novo variants in CLDN5 were identified. In contrast to known causes of AHC, CLDN5 implicates an entirely new disease mechanism – disruptions of the blood-brain barrier.
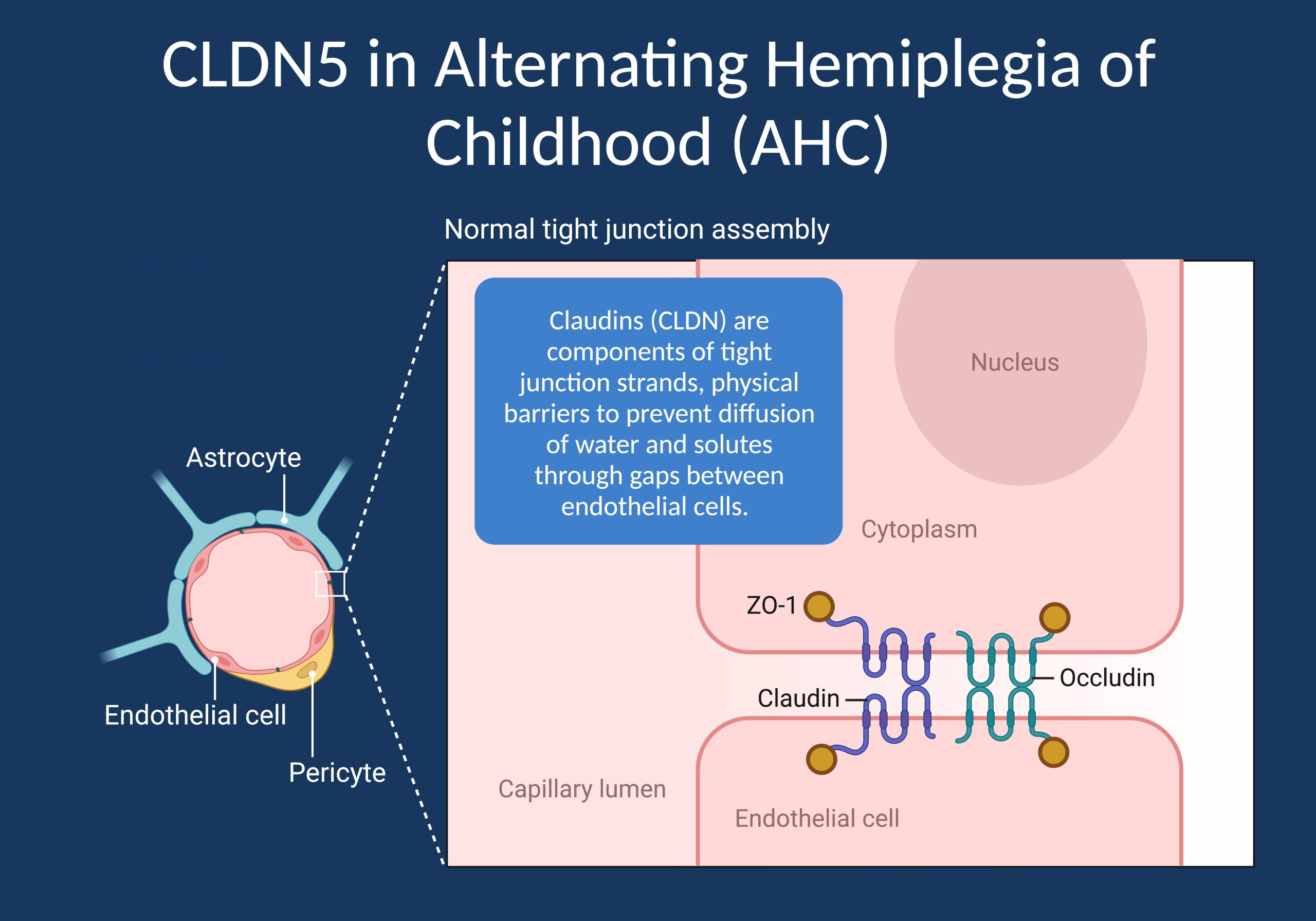
Figure 1. Claudins (CLDN) are components of tight junction strands, which are physical barriers to prevent diffusion of water and solutes through gaps between endothelial cells. In a recent publication, a recurrent de novo variant in CLDN5, the gene coding the most highly expressed tight junction protein in brain endothelial cells, was found to be a novel cause for Alternating Hemiplegia of Childhood (AHC), one of the most mysterious conditions in child neurology [Image created with BioRender].
ATP1A3. We first wrote a blog post on AHC in 2012 when Heintzen and collaborators identified de novo variants in ATP1A3 as the major cause of this condition. Acute hemiplegia in children, i.e., weakness of one side of the body, is always a medical emergency. Causes for a sudden hemiplegia can include intracranial bleeds, tumors, and rare metabolic disorders. Immediate diagnostic work-up is paramount. In some children, no cause is found on brain imaging and extensive testing, and the episode can remit after hours or days. Interestingly, the other side of the body can be affected during a following episode. This condition has been named Alternating Hemiplegia of Childhood (AHC) by Verret and Steele in 1971. AHC is an enigmatic disorder, which is sometimes associated with epilepsy, developmental delay, and dystonia. Even though some mutations in SCN1A, CACNA1A, and ATP1A2 have reported, most cases of AHC are unresolved. Given some resemblance with epilepsy and familial hemiplegic migraine, many children with AHC are followed up by epileptologists.
CLDN5. Tight junctions are critical components of the blood-brain barrier (BBB), a highly specialized structure that tightly regulates the exchange of substances between the blood and the brain. The BBB serves as a barrier to protect the brain from harmful agents, including toxins, pathogens, and certain drugs. Tight junctions play a key role in maintaining the integrity of the BBB by sealing the gaps between the endothelial cells that line the blood vessels in the brain. Claudins are a family of proteins that are essential components of tight junctions, including those found in the blood-brain barrier. These proteins are transmembrane and are responsible for forming the physical barriers that separate the different compartments of cells. There had been no known disease association between claudins and paroxysmal neurological disorders such as AHC or the epilepsies so far. However, this changed with the discovery of CLND5, the gene identified by Hashimoto and collaborators, encoding Claudin-5, one of the most highly expressed proteins in cerebrovascular endothelial cells.
Current study. In their study published in Brain, Hashimoto and collaborators identify a recurrent de novo variant (p.G60R) in two individuals with alternating hemiplegic events and microcephaly. Additional individuals with this recurrent variant and other de novo variants in related phenotypes were found in second study by Deshwar and collaborators, who identify a total of 15 individuals. Many of the individuals in this second study also had alternating hemiplegic attacks. Both individuals reported by Hashimoto and collaborators had recurrent hemiplegic episodes, including episodes provoked by fever. The second individual had a relatively late onset at 30 months, and a follow-up commentary by Panagiotakaki and collaborators raised the issue of whether individuals truly had AHC or a different alternating hemiplegia syndrome, given that some reported features were somewhat atypical. Independent of this discussion, de novo variants in a gene encoding a tight junction protein as a cause for episodic neurological symptoms is unusual. The most well-known BBB disorder is GLUT1 deficiency, where the chronic dysfunction of glucose transport into the CNS causes neurodevelopmental features and seizures. Even though individuals with de novo CLDN5 variants also had other chronic neurological features, the genetic link of BBB dysfunction and acute hemiplegic attacks represents an entirely new disease mechanism.
Disease mechanism. When recurrent variants are identified in genetic disorders, there is always a suspicion of a gain-of-function or dominant-negative effect. In the publication by Hashimoto and collaborators, functional studies showed that the p.G60R variants resulted in a lower sodium and higher chloride permeability of the BBB, essentially turning the protein into an anion-selective channel. However, how these peculiar functional changes are related to hemiplegic attacks and the additional neurological features observed in affected individuals remain unresolved. However, it is clear that the authors have uncovered a previously unknown disease mechanism, linking BBB dysfunction with one of the most poorly understood conditions in child neurology.
What you need to know. Recurrent de novo variants in CLDN5 represent a novel cause for alternating hemiplegia of childhood (AHC). While the proportions of individuals with AHC explained by disease-causing CLND5 variants is likely low, these findings provide a novel insight into the role of the blood-brain-barrier in episodic neurological conditions, which may be the basis for developing new therapeutics.


