Evidence. This week, we will review the evidence that links CACNA1H to human epilepsies. While this gene was initially considered a promising candidate for absence epilepsies, more recent studies have produced little supportive evidence that CACNA1H is linked to human epilepsies. However, CACNA1H may play a role in a different group of diseases, namely early-onset hypertension due to primary aldosteronism. Let’s review what it takes to be candidate gene.
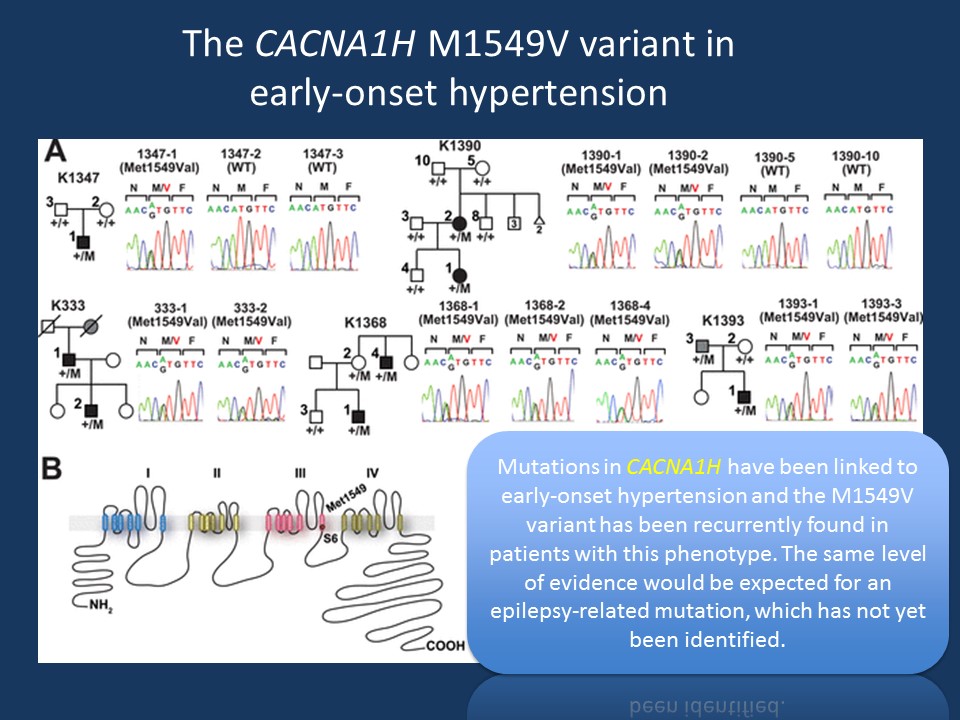
Figure modified from Scholl and collaborators under a Creative Commons Attribution license. Figure legend modified from Scholl and collaborators. (A) Pedigrees of kindreds with CACNA1H M1549V mutation. Studied subjects with early-onset hypertension are shown as black filled symbols, and subjects with early-onset hypertension by family history (K333) or low renin with normal blood pressure (K1393) are shown as grey filled symbols. (B) Transmembrane structure of CaV3.2 (encoded by CACNA1H), the pore-forming subunit of a voltage-gated Ca2+ channel, is shown. These channels have four internal homologous repeats (I–IV), each with six transmembrane segments (S1–S6) and a membrane-associated loop between the pore-forming S5 and S6 segments. The p.Met1549Val mutation is located in S6 of repeat III (modified from http://elifesciences.org/content/4/e06315 under a Creative Commons Attribution license)
Candidate. I would like to start out this blog post by writing about what it takes to be an epilepsy gene. To sum things up, in 2015, any gene faces an enormous uphill battle in order to convince us. This is different from what we thought 10-15 years ago. Back then, we felt that variants in genes not found in controls had a good chance of being disease-related. This was the case for the initial publications in CACNA1H; functional evidence supported a gain-of-function role of CACNA1H variants identified in patients with absence epilepsy. In 2015, we have become much more skeptical. Given the tremendous variability in the human genome, we have realized that a variant’s existence in genes not found in controls does not necessarily mean it is disease-related. There are just so many variants in the human population. The chance of any new variant being benign is much, much higher than being a disease-related variant. There are, however, several ways how we can present a convincing case for involvement of a given gene in disease: replication, recurrence, and unique phenotypes.
Phenotype
Absence Epilepsy. The initial publications on CACNA1H suggested a role for rare variants as risk factors in childhood absence epilepsies (CAE). More recent studies investigating common variants and rare variants do not support a role of CACNA1H variants in CAE. None of the initially reported variants were shown to be de novo or segregating with the disease.
Primary aldosteronism. Rather unexpected, recurrent mutations in CACNA1H have recently been described in early-onset hypertension and primary aldosteronism, an endocrinological disorder. CACNA1H is expressed in the adrenal gland and the mutations were shown to be gain-of-function. In total, Scholl and collaborators identified 5 patients with the identical M1549V mutation, which was shown to be de novo in two patients. None of the patients had epilepsy or neurodevelopmental disorders.
Genotype
CACNA1H in databases. Here is brief overview of what is known about CACNA1H in genome databases. The CACNA1H gene is one of the genes in the human genome that tolerates heterozygous loss-of-function mutations either due to deletions (DGV) or due to loss-of-function mutations (ExAC). Apart from the recurrent M1549V mutation identified in early-onset hypertension, two de novo mutations have been identified in patients with autism and one de novo mutation was identified in an unaffected sibling. We would assume at this point that all de novo mutations other than the recurrent M1549V mutation are incidental findings unrelated to disease.
Epilepsy risk factors. Is it possible that variants in CACNA1H are risk factors for genetic epilepsies and may in fact contribute to disease that we are missing at this point? Yes, but it is difficult for us to make a case for this at this point. We can never say never. All we can say is that we currently do not have the technologies or sample sizes to track down a small effect of CACNA1H variants in human epilepsies. However, the current studies do not provide evidence that the role of CACNA1H is particularly strong.
Mechanism
Hyperexcitability. CACNA1H was initially of interest to the epilepsy community because it codes for a thalamic T-type calcium channel. T-type calcium channels are critical in regulating the excitability level of thalamic pacemaker neurons, which are thought to be involved in the pathogenesis of 3-Hz spike-and-wave associated with generalized absence seizures. The lines of evidence linking CACNA1H to epilepsy in animal models is also indirect. In contrast to other mouse models with calcium channel mutation such as stargazer (CACNG2), lethargic (CACNB4) and ducky (CACNA2D2), CACNA1H mouse models do not present with epilepsy.
Community
There is currently no research community or patient organization for patients carrying CACNA1H variants.


