The 16p11.2 story. Among the various microdeletion and microduplication syndromes located on human chromosome 16, the 16p11.2 microdeletion has unique position. Historically, this microdeletion was the first of the “neurodels” to be identified through association studies in autism, where it can be identified in 0.5% of patients. However, there is more to the phenotypes of the 16p11.2 microdeletion, which is now addressed in a recent paper assessing the full phenotypes in 72 microdeletion carriers. 16p11.2 therefore represents one of the best-investigated microdeletions to date.
The chromosome 16 puzzle. The human chromosome 16 has many segmental duplications, which may serve a default breakpoints for recurrent microdeletions and microduplications. Besides the 16p13.11 microdeletion, the 16p11.2 microdeletion is one of he most prominent of these microdeletions. Weiss and collaborators identified this microdeletion in 2008 through a large multi-center association study, back then a revolutionary way of identifying rare variants using Single Nucleotide Polymorphism (SNP) arrays. This microdeletion was found in 0.5% of patients with autism and currently still represents one of the most frequent autism-related variants. In addition to autistic features, patients with 16p11.2 microdeletions tend to be obese and macrocephalic, i.e. the head circumference is increased. Interestingly, patients with a 16p11.2 duplication have microcephaly and are frequently underweight. This has led Golzio and collaborators to identify KCTD13 as the causative gene for head circumference within this microdeletion. However, the phenotypes of microdeletion carriers is often biased depending on the phenotypes that are investigated within a given study. What are the phenotypes if we take broader look?
The range of 16p11.2 phenotypes. This question was addressed by Zufferey and collaborators in a recent study, which investigated the complete phenotypes of 71 individuals with 16p11.2 microdeletions and their family members. Their study represents one of the few investigations of a large panel of patients with identical microdeletions and gives us the unique possibility to get a deeper insight into the spectrum of microdeletions. Their study came up with three major findings. First, the Full Scale IQ (FSIQ) is lower in microdeletion carriers by two standard deviations. The variability of the FSIQ is similar to controls, which means that that IQ of individuals with 16p11.2 microdeletions can vary on wide range from normal IQ to learning disabilities or intellectual disabilities. Four individuals with 16p11.2 had FSIQ values greater than 100, i.e. their IQ was higher than the population average. Secondly, neuropsychiatric features are common in 80% of individuals, while autism is only present 15%. Third, even though 50% of microdeletion carriers have obesity as a comorbid feature, other dysmorphic features are rare. Epilepsy is seen in 24% of individuals. Taken together, these findings suggest that many of the features seen in 16p11.2 carriers occur on a spectrum that is to some extent indistinguishable for the variation in the general population. This is not what you expect from a disease variant.
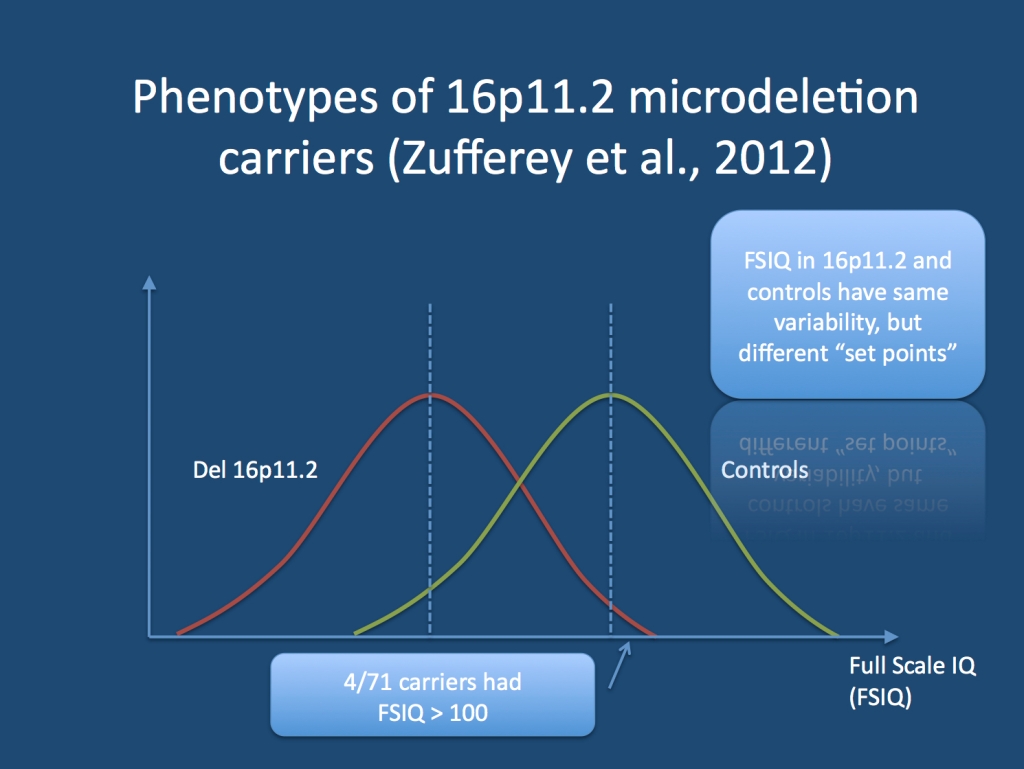
Distribution of Full Scale IQ (FSIQ) values in individuals with 16p11.2 microdeletions and in interfamilial controls. Even though the 16p11.2 carriers have an average FSIQ that is 2 standard deviations lower than controls, there is significant overlap. A subset of 16p11.2 carriers has FSIQ values higher than 100.
Complexity. Many concepts in genetics assume that a disease variant produces a clear phenotype. Concepts such as penetrance imply there is a “black and white” distinction between affected and unaffected. For the 16p11.2 microdeletions, these concepts become increasingly obsolete, as the phenotypes occur on sliding scale. Depending on where the line is drawn between affected and unaffected, the penetrance will vary. Given the distribution of the FSIQ, ~50% of individuals will have intellectual disability. When looking at the distribution, the majority of patients will have FSIQ values in the low normal to mild ID range. This will represent a significant problem for genetic counseling, as most phenotypes are mild, but a significant risk for more severe phenotypes is also present. Also, even though the authors include the observations from family members carrying the 16p11.2 microdeletion, their study cohort is ascertained through individuals with neurodevelopmental disorders. On a population basis, the range of phenotypes might even present less prominently with even more overlap with the general population.
Impact for EuroEPINOMICS. The publication by Zufferey and collaborators suggests that we might rethink our definition of phenotypes. The 16p11.2 microdeletion represents a typical rare variant, where phenotypes appear to occur on a spectrum overlapping with the general population. Similar observations are likely to be made when individuals with 15q13.3, 15q11.2 or 16p13.11 microdeletions are investigated. Epilepsy, however, is a discrete phenotype and additional information including EEG findings on unaffected individuals and information on the phenotypic severity may be useful to assess the spectrum of phenotypes.




Pingback: Gephyrin, the inhibitory synapse and pathogenic microdeletions | Beyond the Ion Channel
Pingback: Dealing with the genetic incidentaloma – the ACMG recommendations on incidental findings in clinical exome and genome sequencing | Beyond the Ion Channel
Pingback: Identifying core phenotypes – epilepsy, ID and recurrent microdeletions | Beyond the Ion Channel
Pingback: Beneath the surface – the role of small inherited CNVs in autism | Beyond the Ion Channel
Pingback: Modifier genes in Dravet Syndrome: where to look and how to find them | Beyond the Ion Channel
Pingback: TBC1D24, DOORS Syndrome, and the unexpected heterogeneity of recessive epilepsies | Beyond the Ion Channel
Pingback: Microcephaly, WDR62, and how to analyze recessive epilepsy families | Beyond the Ion Channel
Pingback: A polygenic trickle of rare disruptive variants in schizophrenia | Beyond the Ion Channel
Pingback: The return of the h-current: HCN1 mutations in atypical Dravet Syndrome | Beyond the Ion Channel