The not so static genome. We usually think that our genome is static and that differences between cell types usually arise through mechanisms that do not necessarily involve alterations of the DNA structure. This suggestion has been challenged by initial data suggesting that retrotransposons may be particulary active in neurones. Now, a recent study in Cell investigates the role of jumping genes using single-cell sequencing of neurons.
Show me all your moves. Transposable elements are part of the DNA that have a life on their own. These elements have the capability of moving from site to site within and sometimes between neurons. Their role within the human genome cannot be underestimated. Almost half of our genome consists of transposable elements. Broadly, these transposons can be subdivided into DNA tranposons and retrotranposons. DNA transposons have the capability to excise themselves from the genome in order to insert somewhere else. While these elements are no longer active in the human genome, they are thought to have been a driving force in primate evolution. Retrotransposons, in contrast, use RNA intermediates for replication and insertion. They can be considered the remnant of retroviruses that now operating privately within the human genome. These retrotransposons fall into two classes: the retrotransposons with long terminal repeats, which closely resemble retroviruses, and the retrotransposons without these repeats. The latter include LINE-1, Alu and SVA elements and account for almost 30% of the human genome. And they appear to be active in the human genome.
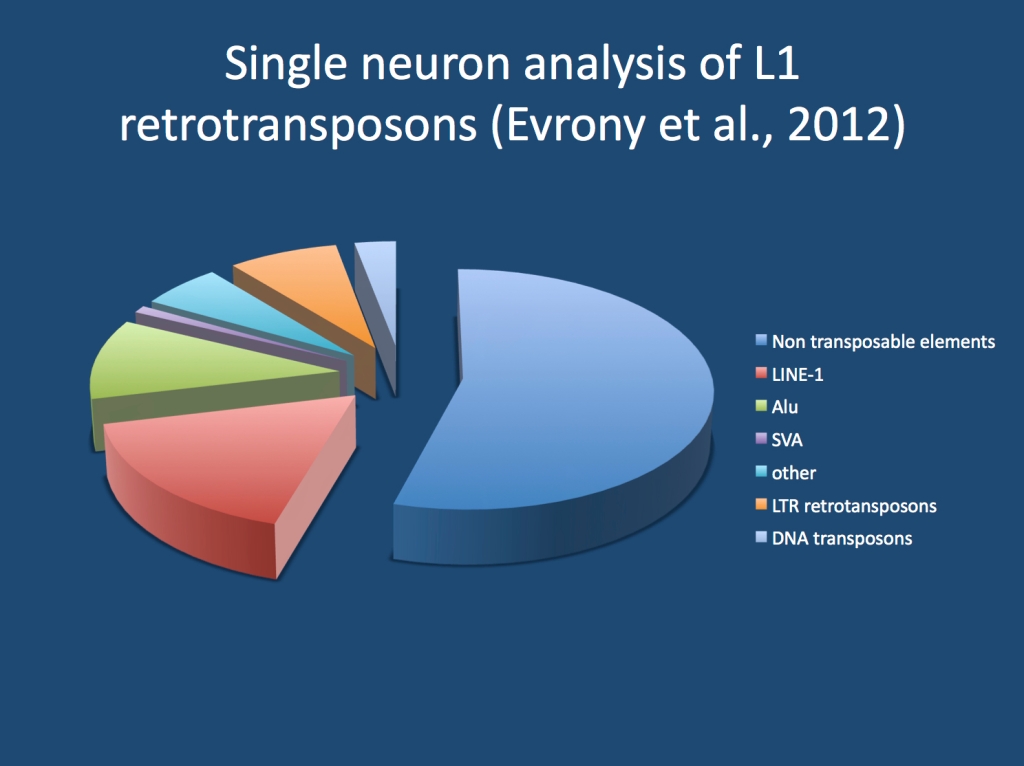
The various mobile elements of the human genome. DNA tranposons and retrotransposons. Amongst the retrotransposons, LINE-1 elements are the most important. LINE-1 elements account for up to 20% of the human genome.
The diversity of neurons. No two neurons are alike, and different neurones in the human brain have different properties and functions. Some authors have suggested that this neuronal diversity might be caused or even be reinforced by somatic mutation mechanisms. Given the activity of human LINE-1 elements, these genomic elements are prime suspects for such a mechanism. Earlier studies in neuronal progenitor cells have suggested up to 80 somatic insertions of LINE-1 elements in each neuron. These insertions could disrupt genes, alter gene expression patterns or even create novel genes depending on the insertion site. However, earlier studies did not look at individual neurons.
Single Neuron Sequencing. Evrony and collaborators have developed a method to sort individual human pyramidal cells using FACS. They have then applied technologies to assess the burden of L1 retrotransposons in 300 human neurons. The authors find that the burden of L1 had been overestimated and that >80% of neurons do not show unique somatic insertions. This observations suggest that L1 elements do not contribute to the diversity of neurons.
Future role or single neuron sequencing studies. In a side-arm of their study, the authors use the same methodology to assess the mosaicism of a somatic AKT3 mutation in hemimegalencephaly. Their method works, and the way is paved for future studies assessing the genomic diversity of neurons. Genome-wide searches for genetic aberrations in tissue is a relatively new field with many important lessons to learn. However, since the area of somatic mutations in epilepsy is not yet addressed, these studies might help us understand the role of somatic mutations and insertions in human epilepsies.



