Ceci n’est pas une pipe. The painter René Magritte was known for his series of paintings that he called The Treachery of Images. He basically painted objects such as pipes, but then felt compelled to point out that the image actually betrays you. It’s not a real pipe, but only an image of it. For some reason, Magritte’s pipe comes to my mind when I read or hear the term Genetic Generalized Epilepsy. Again, the treachery of images. Ceci n’est pas une épilepsie génétique.
I want to believe. Let’s start with a very fundamental and easy question. How do we know that Idiopathic Generalized Epilepsy/Genetic Generalized Epilepsy (GGE/IGE) is genetic? If you look at the literature, you will probably find three lines of evidence including twins, population studies and family studies. The standard argument goes like this: (1) twins provide the strongest evidence for a genetic involvement, (2) population studies demonstrate the applicability to the general patient population and (3) family studies have given us the first genes including GABRG2, GABRA1 and EFHC1. Everything seems to fit nicely together to suggest a strong genetic contribution with a heritability exceeding 80%. There is only one major problem with this: it is not entirely true. We’re taking the upper limit of a range of possibilities and treating this as the ultimate truth. The story of a heritability of 80% and higher fits nicely if you want to believe in a strong genetic aspect, but the heritability might be much, much less. If you feel compelled to pull the plug on your high-throughput sequencer or tear your grant proposal to pieces at this moment, don’t do it just yet. Please keep on reading before you act…
Deconstructing the clinical genetics of GGE/IGE. How much of the evidence for a strong genetic basis to IGE is actually ‘waterproof’? We will soon find that arguments 2 (population studies) and 3 (families) don’t hold. Population studies are usually underpowered to provide us with good heritability estimates, as they rely on comparing the frequency of affected relatives in patients with epilepsy and in controls. Usually, these studies find a higher frequency of affected relatives in patients with epilepsy, which suggests a genetic contribution. However, the margin of error for estimating the size of the genetic load is usually wide, as epilepsy is rare and a positive family history in patients with epilepsy is also rare. Invariably, the numbers that heritability estimates rely on in population studies will be very small. These studies may show that some genetic contribution is present, but it will be difficult to tell whether the heritability is 10%, 50% or 90%. This dilemma has led us to family studies, either through clinical investigations or genetic analysis. Even though family studies may seem ideally suited to investigate the genetic contribution to disease, families are always preselected and cannot tell anything about the genetic contribution in the patient population at large. Even in patients with IGE/GGE, a family history is an exception, not a rule. Gene identification in an epilepsy family suggests a mechanism for how the disease might occur. But even the most successful gene identification in families does not tell us anything about a genetic contribution in sporadic, i.e. non-familial cases. Clinical and genetic findings in families do not provide any evidence that the non-familial cases have any genetic contribution at all.
It comes down to twins – concordant dizygotic twins. With population and family studies out of the race, this leaves us with twin studies. And – at first glance – there appears to a be a very strong argument in favor of a strong, if not exclusive genetic contribution to IGE. Identical twins with IGE are usually concordant, i.e. if one twin is affected, the other twin is affected, as well. In addition, both twins usually have the same epilepsy subtype. Constance and Kathryn, an identical twin pair studies by William Lennox in 1947, are the prime example of this and have become the figureheads of epilepsy genetics. In contrast, the concordance in non-identical twins is much lower, which provides us with a heritability estimate of ~80%. And the study of non-identical twins is exactly where the problem is.
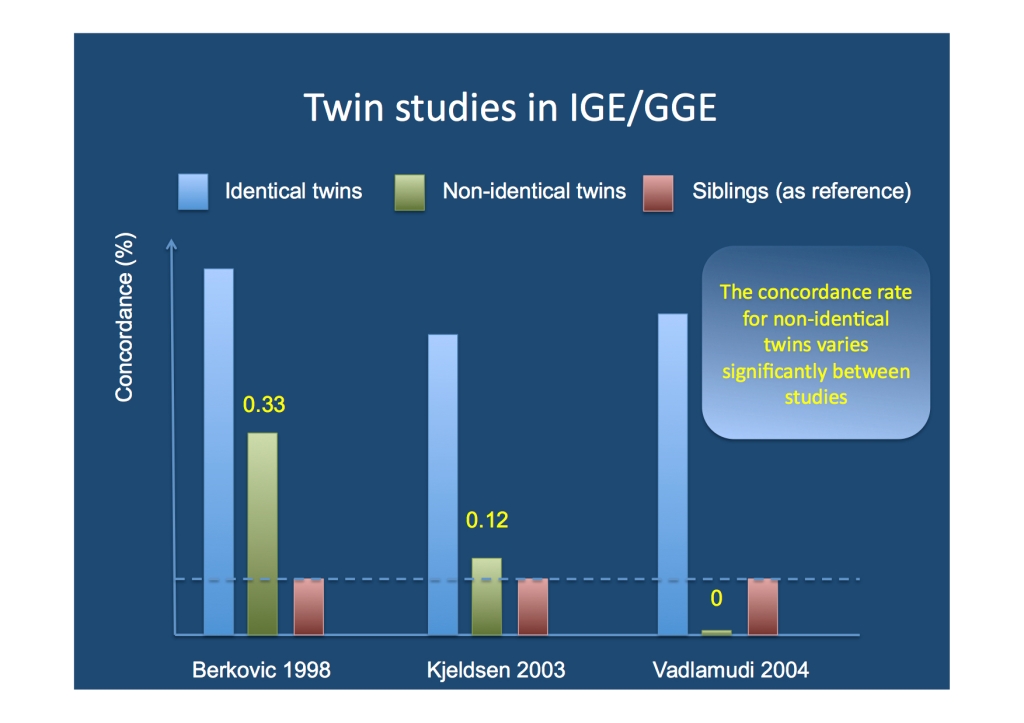
Twin studies in IGE/GGE. While the estimate of concordance in identical twins is virtually the same in all studies, the concordance estimates in non-identical twins vary widely. The heritability of IGE, however, critically depends on reliable estimates for the concordance in non-identical twins.
Concordant non-identical twins: the Achilles’ heel. Recruitment of twins for twin studies often occurs through twin registries and there is an overrepresentation of identical, female twins in these registries. Non-identical twins are usually difficult to ascertain as they might not feel as twin-like as identical twins. There is some truth to this notion: for genetic purposes, non-identical twins are simply siblings born at the same time. Therefore, in a disease that is purely due to genetic factors, they should have the same concordance as siblings, i.e. if one sib is affected with IGE/GGE, the frequency of affected sibs should be same for non-identical twins or siblings. Unfortunately, there is little evidence that this is the case and concordance estimates for non-identical twins vary widely from 0% to 33%. The concordance rate for identical twins is usually constant in existing studies. And the concordance rate of non-identical twins is what really matters.
The shared environment. When the concordance rate in non-identical twins for a disease is much higher than in siblings, the difference cannot be genetic. It must be due to the environment, particularly to the shared environment that twins are exposed to. Much of this is thought to be the in utero environment. Interestingly, identical twins cannot help us distinguish between shared environment or genetic factors; a high concordance in identical twins can be due to both. The distinction between genetic contribution and shared environment can only happen through non-identical twins and the comparison with non-twin siblings. A similar situation has recently shaken the foundations of autism research. A population-based twin study in California has found a strikingly high concordance rate for autism in non-identical twins. This led to a re-appraisal of possible environmental factors and correction of the heritability estimate from 80% down to 30% or less. To put it differently, if the concordance rate in non-identical twins with GGE/IGE is really 33%, shared environmental factors will probably be twice as important as genetic factors and the heritability of IGE will be 20-25%.
Shared environment – a disclaimer. When mentioning “autism” in combination with “environmental factors”, I feel the need to comment on the role of vaccinations, particularly for the non-scientific readers of this blog. In brief, there is no connection. The initial and only study connecting autism to vaccinations in the British Medical Journal was retracted and is considered an elaborate research fraud. Shared environment as suggested by the California autism twin study may refer to known or unknown prenatal conditions and should be a call to action to investigate novel possibly pathogenic mechanisms. Also, there is little evidence that obstetric complications contribute to non-lesional epilepsies.
Why you should keep sequencing. I have tried to highlight why statements like “IGE is almost exclusively genetic” are difficult. It comes down to the concordance frequency in a small group of non-identical concordant twins, and estimates in these groups can be highly biased due to multiple factors. We might never know whether the heritability of IGE/GGE is 20% or 80%, but this does not really matter. The clinical measure of heritability has become almost superfluous and ironically, many diseases with a moderate heritability were the conditions with the highest rate of gene findings. There is much to be discovered in terms of genetic risk factors for GGE/IGE and this work will be entirely independent of whether the heritability is high or low. However, we should not expect genetics to solve the entire puzzle. In past presentations, I introduced IGE/GGE as a “disease that is almost entirely genetic”. I have gotten much more careful about makings these claims.




I appreciate this thoughtful blog of today very much!
Ulrich Stephani
Pingback: Beneath the surface – the role of small inherited CNVs in autism | Beyond the Ion Channel
Pingback: The familial risk of epilepsy – revisited | Beyond the Ion Channel
Pingback: A polygenic trickle of rare disruptive variants in schizophrenia | Beyond the Ion Channel