Angelman Syndrome and UBE3A. Angelman Syndrome is a severe neurodevelopmental disorder characterized by intellectual disability, typical facial features and a usually happy demeanor. Patients with Angelman Syndrome usually do not acquire active speech and often show a characteristic, atactic gait. Also, patients with Angelman Syndrome have a characteristic EEG pattern and many children have seizures. Angelman Syndrome is a genetic disorder due to loss of function of UBE3A, a ubiquitin ligase expressed in the CNS. Ubiquitin ligases are the bin collectors of the cell. By attaching ubiquitin to proteins, proteins are labelled for cellular degradation. How a malfunction of a cellular garbage truck causes such a complex neurodevelopmental disorder is poorly understood. A recent study, however, points out an important role for interneurons….
Microdeletions, mutations, UPD. The genetics of Angelman Syndrome (AS) is anything else than straightforward – AS can be caused by microdeletions, mutation and paternal uniparental disomy, i.e. the inheritance of both chromosomes from the father. The common denominator is a lack of UBE3A expression. UBE3A is a one of the imprinted genes of the human genome, i.e. whether this gene is expressed depends on the parent who transmitted the chromosome containing UBE3A. If the chromosome or chromosomal region comes from the mother, the gene is expressed. However, it is silent when this gene is transmitted from the father. Consequently, when the UBE3A copy coming from the mother carries a mutation of deletion, the paternal copy cannot compensate – UBE3A is not expressed, even though the paternal copy is still present in the genome. Likewise, if both chromosomes are transmitted from the father, UBE3A is also not expressed.
Hyper- or hypoexcitability. There is not much known about how UBE3A leads to neuronal dysfunction. Now, a study by Wallace and colleagues investigated the differences in various neuronal cell types using a mouse model of AS. Interestingly, this mouse model also develops AS-like features when the maternal allele is not present or is mutated. Paradoxically, they find that the transmission of excitatory synapses is impaired, a finding that has previously also been reported by others. Even though this finding might explain parts of the intellectual disability, a lack excitation would not fit with the EEG and seizures that many AS patients have. Therefore, Wallace and colleagues looked further…
Interneuron cycling. In addition to a defect in excitatory synapses, Wallace and colleagues also found that the transmission of GABAergic synapses is impaired. This impairment is particularly obvious when synapses recover from fast activity and vesicle depletion. In patients with AS, synapses in GABAergic interneurons recover much slower from depletion, creating a net excitation because of the excitatory synapses that recover faster. This suggests that some of the pathology in AS is due to defective vesicle cycling in the brain.
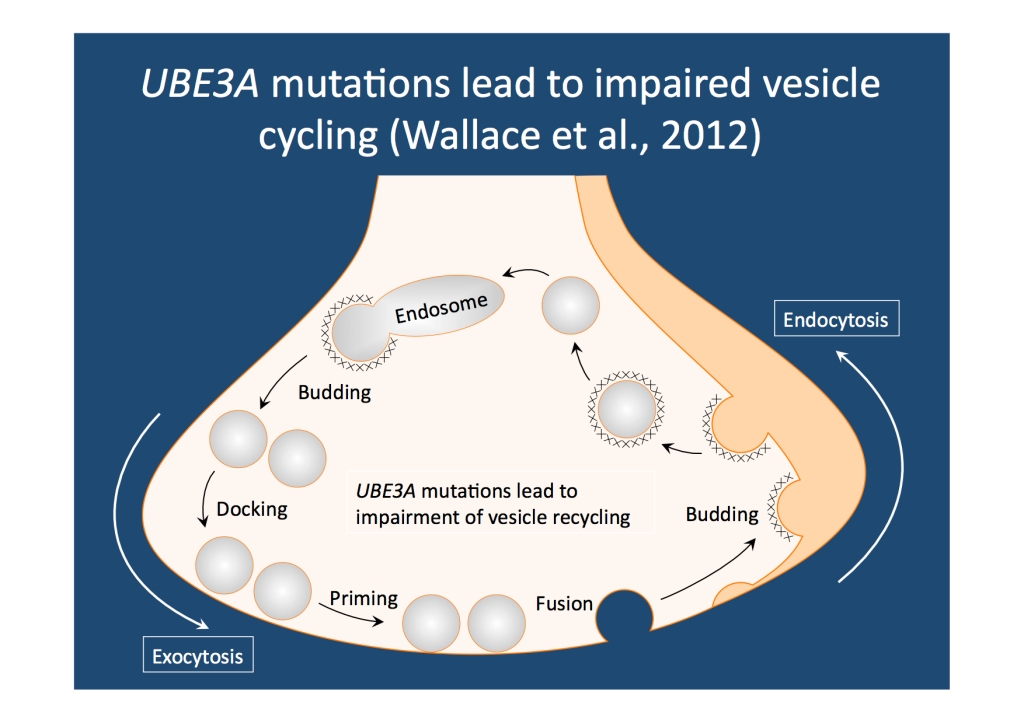
Recycling synaptic vesicles. UBE3A is important for rapid vesicle recovery. Furthermore, UBE3A is more important in interneurons than in pyramidal cells, explaining the dysregulation between GABAergic and glutamateric synapses in the CNS.
EuroEPINOMICS and interneurons. In contrast to pyramidal cells, interneurons are highly heterogeneous, divided into different groups based on location, staining for specific proteins and electrophysiological properties. By connecting the network defects in AS back to deficits in interneuron transmission, the term interneuronopathy comes to mind. Many mutations involved in CNS disorders result in interneuron dysfunction including ARX and SCN1A. Keeping in mind that neurons in the brain can be very different will help us make sense of some of the mutations we find in EuroEPINOMICS.



